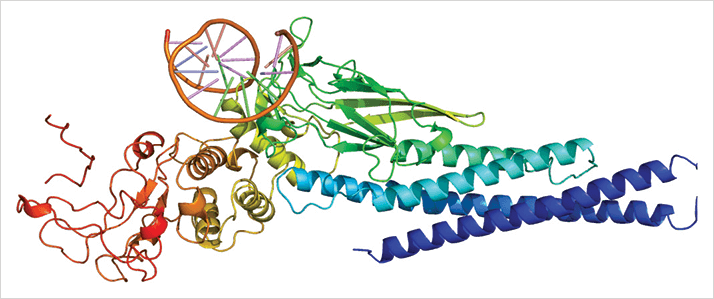
Macrophages are involved in many of the pathological processes that occur as people age. In diseases that involve angiogenesis (like wet AMD), it’s the M2 macrophage type (which is usually characterized as a tissue-repairing cell) that’s associated with this process, as it has the ability to promote angiogenesis (1). Previous studies have noted that both M2 macrophages and the level of a specific cytokine, interleukin-10 (IL-10), are elevated in aging eyes before macular degeneration leads to detectable vision loss – but until now, researchers haven’t known why. Researchers at the laboratory of Rajendra Apte at the Washington University School of Medicine, St. Louis, USA may have the answer to that question (2). Their study reveals a particular pathway in senescent eyes, wherein IL-10 activates a signaling molecule known as STAT3 (Figure 1). The signal from STAT3 induces the alternative (macrophage) activation pathway, generating a particular M2 macrophage subtype that leads to pathological choroidal neovascularization. But the researchers didn’t stop there – after establishing IL-10 and STAT3 as key regulators of M2 macrophage presence in the eye, they investigated the possibility of targeting those molecules to reduce macrophage presence and neovascularization.
Mice treated with an antibody designed to block the IL-10 receptor showed a fivefold reduction in vascular proliferation compared with control mice, a finding echoed when the researchers examined mice with a genetic knockout of the receptor. Furthermore, macrophages from the transgenic mice showed nearly double the ability of those in normal mice to inhibit endothelial cell proliferation in cell culture, and also had a higher proportion of M1 macrophage markers, indicating that the macrophages in those mice were less M2-like – possibly due to the absence of IL-10/STAT3 signaling. To test the function of the STAT3 molecule, the researchers created a new “floxed” transgenic mouse whose myeloid cells (including macrophages) lack functional STAT3. That deficiency, like the IL-10 receptor knockout, inhibited the induction of M2 macrophage markers – so the researchers tried injecting the STAT3-deficient macrophages directly into the eye. Mice receiving those intravitreal injections displayed significantly less choroidal neovascularization than those receiving control macrophages.
Apte’s team went on to investigate the role of STAT3 in the pathogenesis of wet AMD in humans. Western blot analyses of human peripheral blood mononuclear cells (PBMCs) isolated from patients with AMD (and compared with PBMCs from non-AMD age-matched controls) revealed STAT3 expression was significantly greater in patients with AMD than those without, and immunohistochemistry showed that the molecule was associated with M2 macrophages in the choroidal neovascular membranes of patients with AMD. This research raises the hope that compounds that successfully target STAT3 in mice may one day yield a therapeutic option for humans with AMD.
References
- N Jetten, et al., “Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo”, Angiogenesis, 17, 109–118 (2014). PMID: 24013945. R Nakamura, et al., “IL10-driven STAT3 signalling in senescent macrophages promotes pathological eye angiogenesis”, Nat Commun, 6, 7847 (2015). PMID: 26260587.
