Are we finally on the cusp of treating neurodegenerative ocular disease by neuroprotection? Currently, IOP is the only modifiable risk factor for glaucoma, but today’s treatments – despite their successes in reducing IOP – are not always effective, as many patients continue to experience gradual loss in vision (albeit at a less dramatic pace) with treatment. Whilst efforts in the glaucoma field have been focusing on neuroprotective treatments, so far these have been to little avail (1). Enter Hanako Ikeda and her team at Kyoto University. Two years ago, they published research showing that their novel neuroprotectant compounds – termed Kyoto University Substances (KUSs) – prevented photoreceptor cell death and preserved visual function in a mouse model of retinitis pigmentosa (2). Now they have shown that KUSs can prevent glaucoma progression and loss of visual function in three different mouse models, through suppressing the loss of retinal ganglion cells (RGCs) and nerve fibers (3). But what exactly are KUSs and how do they work?
Based on the structure of a naphthalene derivative, KUSs target valosin containing protein (VCP), an intracellular protein involved in a plethora of cellular processes (2). Linked to several polyglutamine neurodegenerative diseases, VCP has also been identified as the causative gene in inclusion body myopathy with Paget disease of bone and frontotemporal dementia and rare cases of ALS/Lou Gehrig’s disease (4,5). Interestingly, mutated VCP proteins exhibit elevated ATPase activities which correlate with clinical severity of disease (6). KUSs inhibit this ATPase activity of VCP and maintain cellular ATP levels (2,6). What makes VCP interesting for ophthalmology is its high expression in all types of retinal neuronal cells and especially RGCs – the casualties in glaucoma. Ikeda’s team have shown that KUSs exhibit neuroprotective effects on these cells by preventing ER stress and cell death, thereby mitigating disease progression in mouse models of glaucoma. Ikeda notes that “from our cellular studies, we expected that KUSs would protect RGCs to some extent in these glaucoma models [...] we were pleasantly surprised that we could confirm their functionality by electroretinograms”.
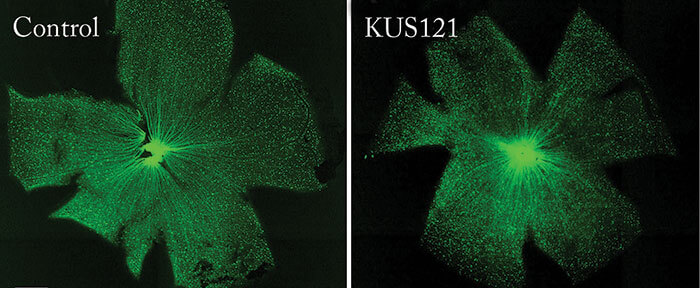
The team also showed that NMDA-induced RGC injury in thy1-CFP transgenic mice – which express cyan fluorescent protein (CFP) in RGCs – could be rescued by preceding KUS treatment. Ikeda comments, “it was a challenge to accurately measure the remaining RGC numbers through disease progression or treatment. To meet this challenge, we customized our OCT instrument (for the mouse) to detect CFP and used thy1-CFP transgenic mice […] this approach allowed us to take live images of fluorescing RGCs and nerve fibers in our mouse models of glaucoma. We successfully used this instrument in our mouse studies to evaluate the extent of retinal damage, as doctors do for patients.” So what are the next steps? Ikeda says, “we believe that a more convenient and ideal administration route is via eye drops. We will modify the dosage forms of KUSs for eye drops, and we will then examine their efficacies in our mouse models of glaucoma and retinitis pigmentosa.” Ikeda reveals that they are also planning Phase I/II clinical trials to test the safety and efficacy of KUSs administered through intravitreal injection, but estimates that it will take at least another three to five years for glaucoma studies, for which much longer term safety tests will be needed. Hopefully glaucoma treatment is on the cusp of something exciting.
References
- RN Weinreb et al., “The pathophysiology and treatment of glaucoma: a review”, JAMA, 14, 1901–1911 (2014). PMID: 24825645. HO Ikeda et al., “Novel VCP modulators mitigate major pathologies of rd10, a mouse model of retinitis pigmentosa”, Sci Rep, 6, 5970 (2014). PMID: 25096051. N Nakano et al., “Neuroprotective effects of VCP modulators in mouse models of glaucoma”, Heliyon, 2, e00096 (2016). GD Watts et al., “Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein”, Nat Genet, 26, 377–381 (2004). PMID: 15034582. JO Johnson et al., “Exome sequencing reveals VCP mutations as a cause of familial ALS”, Neuron, 9, 857–864 (2010). PMID: 21145000. A Manno et al., “Enhanced ATPase activities as a primary defect of mutant valosin-containing proteins that cause inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia”, Genes Cells, 15, 911–922 (2010). PMID: 20604808.
