RTH258. “Son of Lucentis.” The drug is brolucizumab – a single-chain antibody fragment, a potent inhibitor of VEGF, and it’s claimed to be “the smallest known active unit of an antibody.” It also holds the potential of a 12-weekly treatment interval for the treatment of AMD, which, if true, clearly means fewer hospital visits for patients and an easing of the burden for the healthcare professionals that have to run the clinics. Last month, at the American Academy of Ophthalmology annual meeting in New Orleans, Pravin Dugel presented the much-anticipated 48-week data from the 96-week, Phase III evaluation of two doses of the intravitreally-administered anti-VEGF drug compared with aflibercept for the treatment of neovascular AMD in the HAWK and HARRIER trials (1).
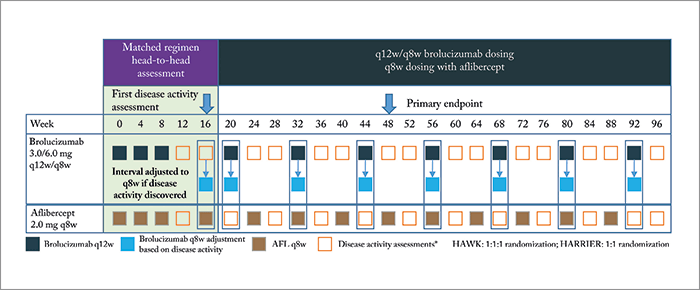
The trial design, treatment regimens and disease activity assessments are depicted in Figure 1, and consisted of two phases: an initial 16-week matched regimen period to provide a head-to-head comparison of brolucizumab (3.0 or 6.0 mg) and aflibercept 2.0 mg, which was followed by brolucizumab being dosed in 12-weekly intervals (q12w) – reduced to 8-weekly intervals (q8w), if signs of disease activity were noted. Patients randomized to receive aflibercept were administered q8w doses for the rest of the study.
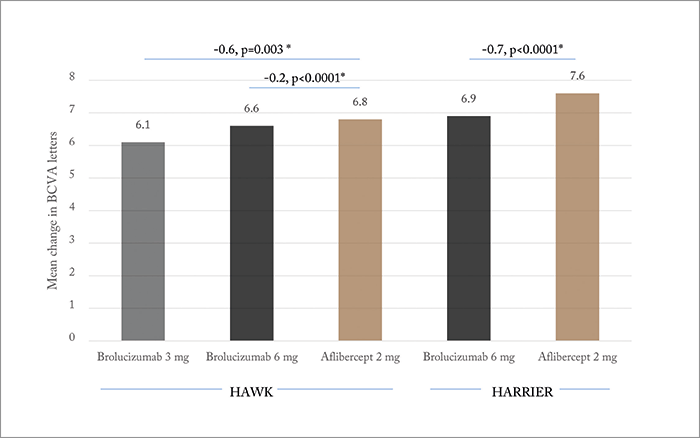
So what does the 48-week data show? Brolucizumab met the primary (non-inferiority) endpoint: change in BCVA from baseline to week 48 (Figure 2). Not all patients were able to maintain the q12w brolucizumab treatment interval – in HAWK, 52 and 57 percent of patients remained on q12w for the 3.0 and 6.0 mg doses, respectively. And in HARRIER (which evaluated only the 6.0 mg brolucizumab dose), 52 percent of patients remained on the longer interval by week 48. However, the visual gains achieved by all drugs and dosing arms were robust in the head-to-head assessment period, and were maintained out to week 48. Additionally, significantly (p<0.05) fewer brolucizumab-receiving patients displayed disease activity at week 16 (HAWK: 27.4, 23.5 and 33.5 percent for brolucizumab 3.0 mg, 6.0 mg and aflibercept 2.0 mg, respectively; HARRIER: 21.9 vs. 31.4 percent). In both trials, the novel agent was also found to have achieved superior reductions in central subfield thickness measurements in both the head-to-head and maintenance phases, and significantly fewer brolucizumab-receiving patients had intraretinal, subretinal or sub-RPE fluid on assessment at week 16 or 48. In terms of safety, ocular, non-ocular and serious adverse event rates were similar across treatment groups. Can brolucizumab stretch those treatment intervals out beyond what’s currently achievable? On the basis of the HAWK/HARRIER trial data, it seems that for some patients, at least, the answer could be “yes.” Dugel stated in his presentation that further details of the HAWK and HARRIER trials will be presented in future congresses – and it was clear that those details are keenly awaited.
Though the HAWK and HARRIER studies (1) provided compelling evidence of a potentially significant biologic effect of brolucizumab for patients with neovascular AMD, there are some preliminary concerns with respect to the way the data were presented. For one, the proportion of patients who completed the study protocol was not shown – as one would normally expect to see in an initial presentation of pivotal clinical trial data. This is particularly relevant to these studies with their complicated treatment regimen assignments for the brolucizumab cohorts. As a result, it is unclear how many subjects may have been exited from the studies (in each of the cohorts) as a result of needing treatment more frequently than every eight weeks. Because we don’t know how many subjects were exited for this reason (or for other reasons), it is difficult to interpret the potential impact this may have had on the reported treatment effect. Furthermore, the data were not presented in a way that allows one to discern the treatment effect of the brolucizumab cohort dosing strategies. Rather, we were left only to draw efficacy and safety conclusions on the basis of pooled brolucizumab data with their varied dose and treatment frequency regimens. Andrew Moshfeghi serves as Associate Professor and Director of the Clinical Trials Unit for the Department of Ophthalmology at the USC Eye Institute.
References
- PU Dugel et al., “HAWK & HARRIER: 48-week Results Of 2 Multi-Centered, Randomized, Double-Masked Trials of Brolucizumab Versus Aflibercept For Neovascular AMD”, presented at: The American Academy of Ophthalmology 2017 Annual Meeting on November 10, 2017, New Orleans.
