
Nearly every glaucoma patient on topical eye drops shows some form of ocular surface compromise, yet this critical issue is often overlooked. While studies report that 48–59% of these patients experience symptoms and 22–78% show clinical signs of ocular surface disease (OSD) (1), my experience suggests this issue is even more pervasive.
Dry eye disease is a multifactorial condition characterized by loss of tear film homeostasis due to tear film instability, hyperosmolarity, ocular surface inflammation, and neurosensory abnormalities (2). In glaucoma patients, these mechanisms are frequently exacerbated by the chronic use of topical medications.
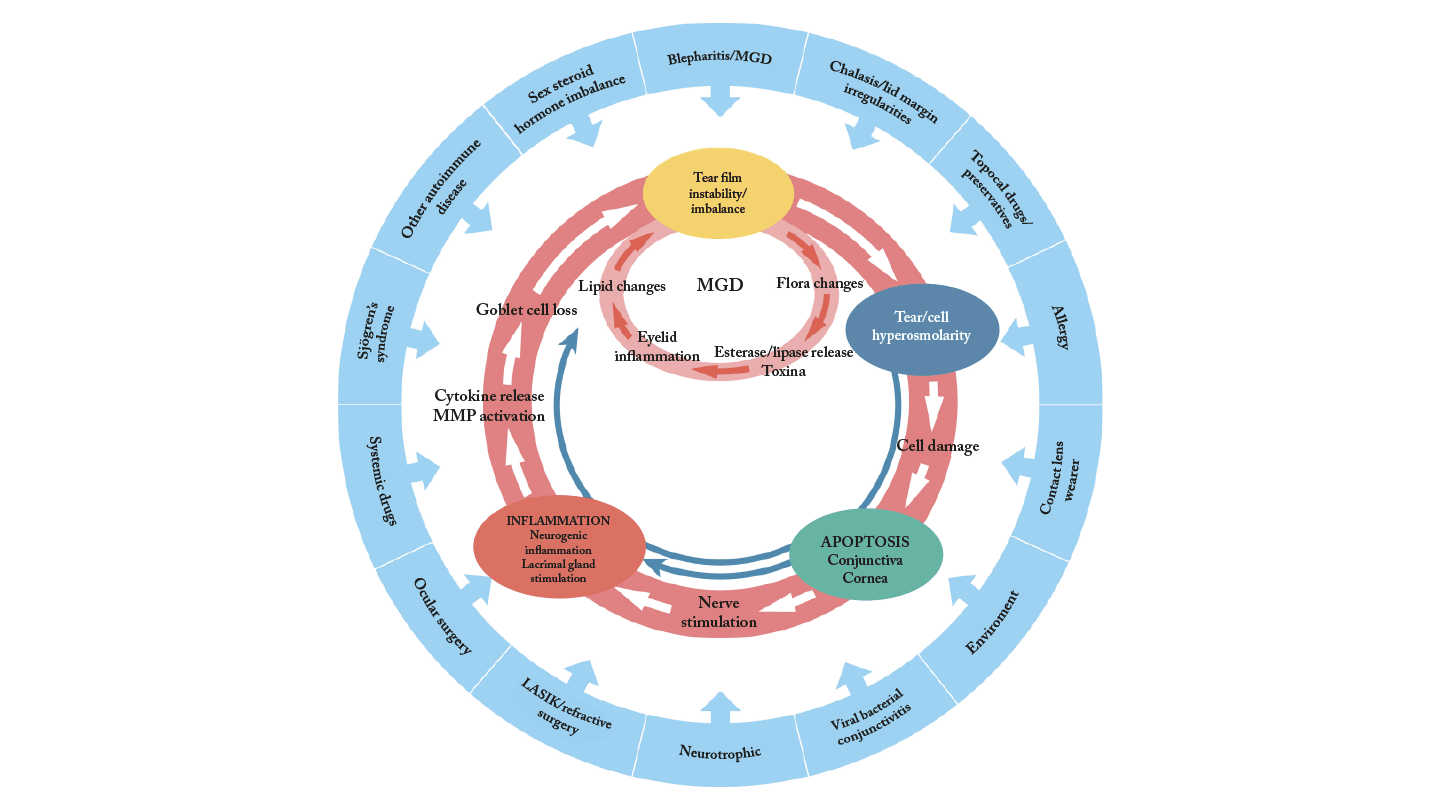
These patients may present with aqueous deficiency and evaporative dry eye but, critically, also mucus deficiency, which I believe is the defining feature of glaucoma-related dry eye (3). Many of these patients fall into the category of “decreased wettability dry eye” (3). Given this, if I were to choose just one diagnostic test for glaucoma patients with dry eye, it would be tear breakup time (TBUT), which is a simple measure of tear film stability.
The vicious cycle of OSD in glaucoma
More than just a co-morbidity, dry eye disease is a key driver of poor outcomes in glaucoma. The complex interplay between chronic glaucoma therapy and OSD creates a vicious cycle that affects intraocular pressure (IOP) control, reduces patient adherence, compromises surgical outcomes, and negatively impacts quality of life (4). Multiple medications increase cumulative exposure to toxic compounds, leading to deeper penetration of these toxic compounds into ocular tissues, increased trabecular meshwork inflammation, and higher aqueous outflow resistance. This in turn increases the treatment burden and drives a need for surgery, which itself may be compromised by OSD (4).
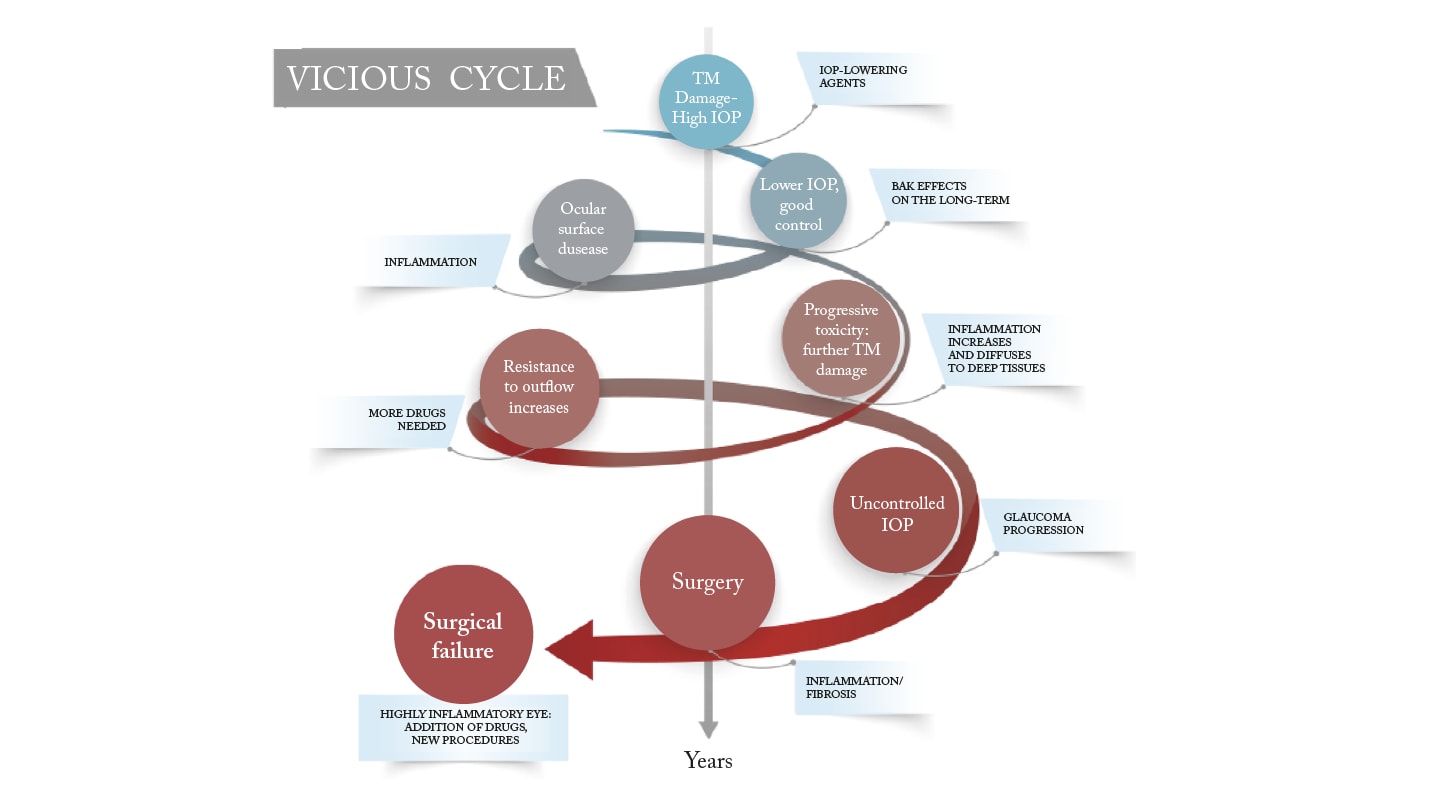
Every class of topical IOP-lowering therapy can induce or worsen OSD. This may be due to the active ingredient, excipients, or most often, the preservatives. If I had to eliminate one class from the standpoint of ocular surface health, it would be the alpha-adrenergic agonists, which tend to be particularly poorly tolerated.
Why addressing OSD matters in glaucoma management
DED causes significant patient discomfort, including redness, burning, stinging, fluctuating vision, foreign body sensation, photophobia, itching, and eye fatigue. These symptoms impair work productivity, limit visual activities, reduce vision-related social function, and affect both general and mental health. In short, DED significantly lowers quality of life, which explains why many glaucoma patients are “unhappy” even when their disease seems well-controlled.
Ocular discomfort and poor tolerability are major reasons for non-adherence. One study found that only 50% of patients remained on treatment after 6 months, with just 37% of cases persisting at 3 years (5). Poor adherence results in poor IOP control and jeopardizes long-term visual outcomes. Furthermore, chronic ocular surface inflammation can reduce the efficacy of medical therapy and increase the risk of surgical failure.
Breaking the cycle: Focus on what we can change
The key to managing patients with coexisting glaucoma and DED is to focus on modifiable factors. This requires a fundamental shift in how we approach the glaucoma care pathway, starting from the earliest stages of disease management.
Step 1 is to evaluate every patient for OSD prior to initiating glaucoma therapy. This means going beyond a routine history to include formal symptom assessment and clinical evaluation. Clinicians should document risk factors such as age, systemic disease, previous ocular surgery, and environmental exposures. Pain scales can aid in detecting corneal innervation abnormalities, especially when symptoms appear disproportionate to clinical signs. At the slit lamp, TBUT should be assessed and blink patterns evaluated. If OSD is diagnosed or significant risk factors identified, clinicians should consider initiating treatment with preservative-free topical medications, or offering selective laser trabeculoplasty (SLT) as a first-line option to reduce the risk of triggering or worsening ocular surface damage.
There are several tear substitutes available, but I would recommend using a preservative-free, non-aqueous, multiple-action formulation that addresses the complex mechanisms of dry eye in glaucoma patients. Options include sodium hyaluronate, carbomer, and triglycerides (such as Artelac Complete™ or Neraya™), or more viscous combinations like sodium hyaluronate, trehalose, fucoidan, and D-panthenol (Artelac Ultra 4S™ or RecuDrop™). For patients requiring even greater ocular surface protection and longer retention, carbomer 0.3% with D-panthenol (Cornelegel™ or RecuGel™) can be considered, particularly in cases with significant epithelial damage or nocturnal discomfort.
Step 2 involves re-evaluating the patient for signs of OSD regularly throughout glaucoma treatment. It is well established that the prevalence of OSD increases with patient age, number of medications, and treatment duration. Monitoring should extend beyond symptoms to encompass adherence, quality of life, and functional vision. Therapy adjustments should be proactive, not reactive.
Step 3 focuses on situations where OSD begins to interfere with glaucoma outcomes. At this stage, clinicians must identify the primary mechanisms driving ocular surface dysfunction. Potential irritants, particularly preservatives, should be removed. Strategies such as switching to fixed combinations to reduce drop burden, offering laser therapy or minimally invasive surgery, or prescribing adjunctive OSD therapy with preservative-free tear substitutes become essential. In some cases, anti-inflammatory agents like tacrolimus or cyclosporine may be used as steroid-sparing options.
Step 4 is relevant when surgery becomes necessary due to poor IOP control or drop intolerance. Preoperative ocular surface optimization is critical. Inflammatory load should be reduced by discontinuing topical IOP-lowering drops where possible and initiating anti-inflammatory therapy. Minimally invasive procedures, such as excimer laser trabeculostomy (ELT), may be preferred for milder disease due to their more favorable impact on the ocular surface. Postoperative care is equally important, particularly after filtration surgery, as bleb-related inflammation and discomfort may cause further epithelial compromise. In advanced cases with significant epithelial compromise, autologous plasma-rich growth factors (PRGF) may be considered.
Importantly, we now understand thatpreservative-induced fibrosis is not limited to the ocular surfaceand can also affect the trabecular meshwork, episcleral tissue, and uveoscleral outflow pathway, thereby contributing to elevated IOP and poorer surgical outcomes. Normally, epithelial cells express primary cilia that act as pressure sensors. In a fibrotic state, these cilia are lost, altering the mechanosensitivity of the tissue and disrupting physiological aqueous outflow. Therapies such as PRGF may help restore these structures, offering a novel strategy to allow epithelial regeneration with functional cilia.
This article is based on a lecture presented at the Bausch + Lomb Glaucoma Summit 2025, held in Baveno, Italy (May 22, 2025).
References
- OE Kemer et al., "Managing Ocular Surface Disease in Glaucoma Treatment: A Systematic Review," Bioengineering,11, 1010 (2024). PMID: 39451386.
- C Baudouin et al., "Role of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meeting," Ocul Surf., 11, 246 (2013). PMID: 24112228.
- JS Wolffsohn et al., "TFOS DEWS III Diagnostic Methodology," Am J Ophthalmol., online ahead of print (2025). PMID: 40451408.
- C Baudouin et al., "Inflammation in Glaucoma: From the back to the front of the eye, and beyond," Prog Retin Eye Res., 83,100916 (2021). PMID: 33075485.
- G Li et al., "Glaucoma and Ocular Surface Disease: More than Meets the Eye," Clin Ophthalmol.,16, 3641-9 (2022). PMID: 36389640.
